Cutera’s AviClear Gains FDA Approval as a Groundbreaking Long-Term Acne Treatment
Cutera’s revolutionary treatment, AviClear , has received clearance from the U.S. Food and Drug Administration (FDA) as a long-term solution for mild to severe inflammatory acne vulgaris.
In March 2022, AviClear initially obtained FDA clearance for acne treatment. However, the recent FDA approval now recognizes AviClear as the first acne therapy to provide long-term effectiveness for mild, moderate, and severe acne.
Dermatologists, Master Estheticians and patients alike are astounded by this achievement. Dr. Michael H. Gold, Medical Director of Gold Skin Care Center, expressed his enthusiasm, stating, “It is incredibly impressive that AviClear is now the first and only acne therapy, of any kind, able to claim long-term effectiveness for mild to severe acne.” He further emphasized that this is a significant breakthrough for patients seeking reliable long-term clearance among the various treatment options available.
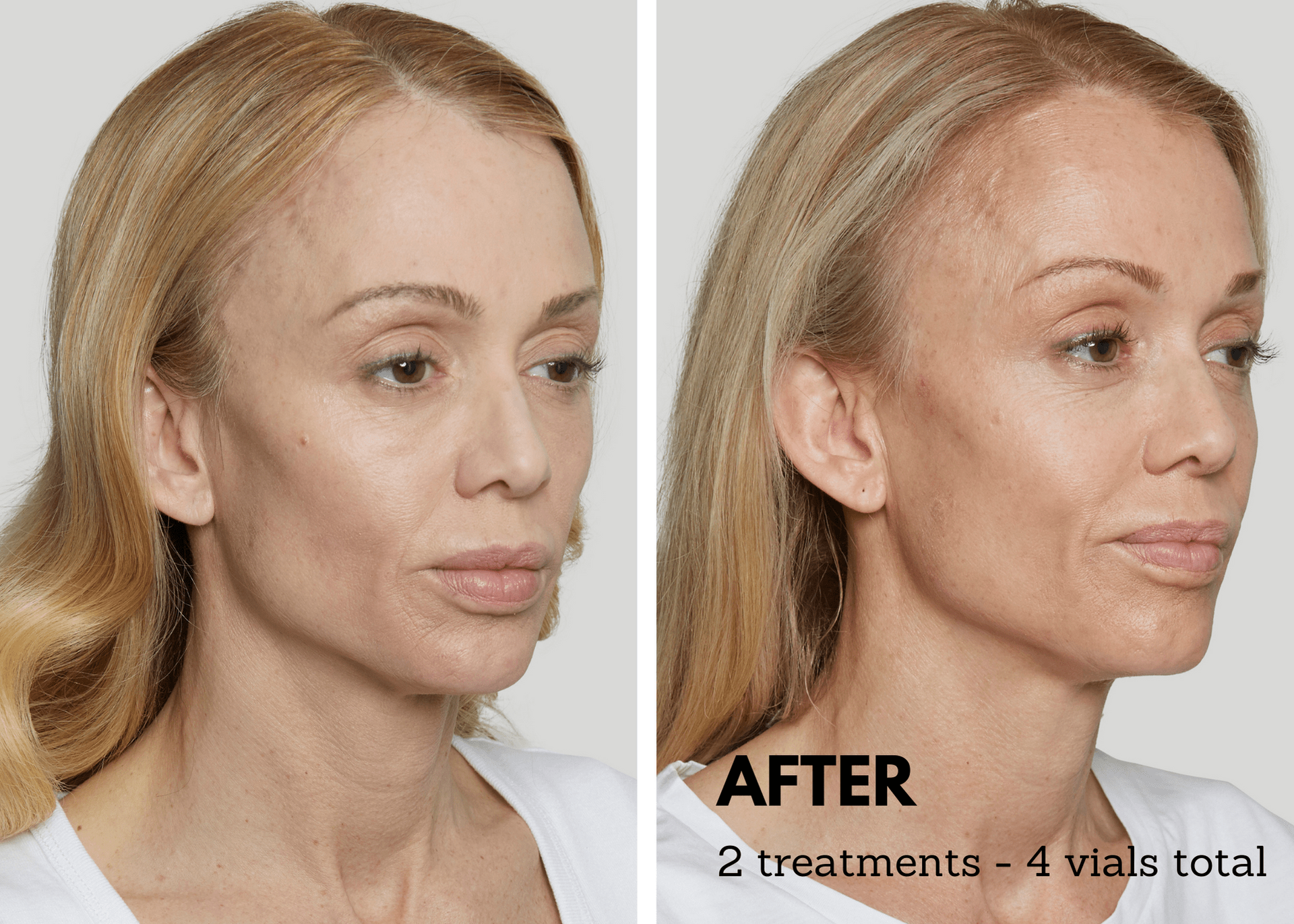
AviClear, the pioneering 1726nm laser on the market, specifically targets and suppresses sebaceous glands, effectively eliminating acne at its root cause. Patients and providers now have access to a durable, prescription-free alternative. After three 30-minute treatment sessions, 90% of patients witnessed visible improvement in their acne six months following their final session. Clinical data over a 12-month period confirms that improvement increases to an impressive 92%, solidifying the long-term efficacy of AviClear in clearing acne and enhancing skin quality over time.
Master Estheticians, Shelly Snodgrass and Amanda McLaughlin, here at Camas Medspa in Vancouver, WA share in the excitement “We, as practitioners who have been administering AviClear to our patients since March of 2022, have observed a remarkable trend: the treatment’s results continually improve over time.”
Their delight is further emphasized they express their happiness in the FDA’s recognition of these enduring outcomes, which instills a profound sense of confidence among both patients and providers regarding the remarkable effectiveness and long-lasting durability of AviClear’s results.
Sheila A. Hopkins, Interim CEO at Cutera, proudly acknowledges this significant milestone, saying, “We are proud to receive such a significant and landmark designation. The success of AviClear is a testament to Cutera’s ingenuity and innovation as a pioneering force in results-driven technology.” Hopkins emphasizes Cutera’s commitment to developing breakthrough treatment options, exemplified by AviClear, as the company celebrates its 25-year history of groundbreaking devices.
With FDA approval as a long-term acne treatment, AviClear sets a new standard in addressing acne concerns and offering patients renewed confidence in their skincare journey.
The post Cutera’s AviClear Gains FDA Approval as a Groundbreaking Long-Term Acne Treatment appeared first on Camas Medspa.